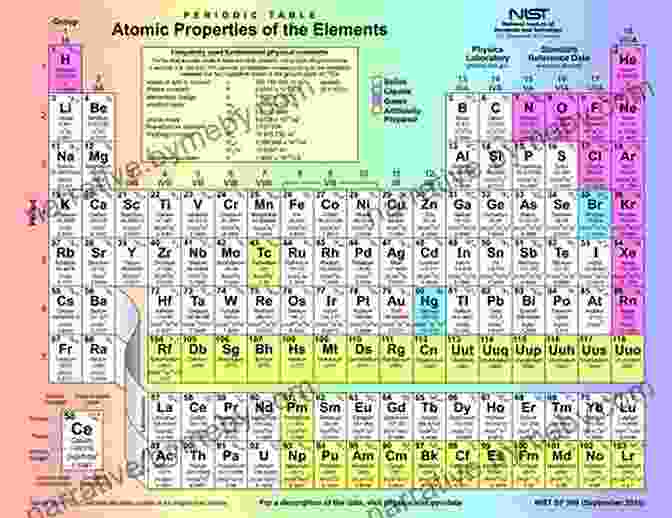
An Introduction to the Periodic Table of Elements: A Comprehensive Guide to the Essential Building Blocks of the Universe

: The Rosetta Stone of Chemistry
The periodic table is a visually stunning and intellectually stimulating arrangement of chemical elements that serves as a roadmap to the structure, properties, and reactivity of matter. It's a powerful tool that has revolutionized our understanding of the natural world, making it indispensable for chemists, physicists, biologists, and anyone interested in the fundamental workings of the universe.
4.1 out of 5
| Language | : | English |
| File size | : | 3515 KB |
| Print length | : | 66 pages |
Navigating the Periodic Table
The periodic table is organized into 18 vertical columns, known as groups, and 7 horizontal rows, called periods. Elements are arranged in Free Download of increasing atomic number, which is the number of protons in the nucleus of an atom. The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom.
Elements in the same group share similar chemical properties because they have the same number of valence electrons, which are the electrons in the outermost energy level of an atom. Elements in the same period have the same number of electron shells, which are the energy levels around the nucleus.
Exploring the Elements
Each element in the periodic table has a unique set of properties that determine its behavior in chemical reactions. These properties include:
- Atomic number: The number of protons in the nucleus.
- Atomic mass: The average mass of an atom of the element, taking into account the isotopes.
- Electron configuration: The arrangement of electrons in the energy levels around the nucleus.
- Oxidation state: The number of electrons that an atom can gain or lose in a chemical reaction.
- Reactivity: The tendency of an element to participate in chemical reactions.
The periodic table is a treasure trove of information about the elements. It allows us to predict the properties of new elements, understand the behavior of atoms in chemical reactions, and design new materials with specific properties.
Applications of the Periodic Table
The periodic table has countless applications in various fields, including:
- Chemistry: Understanding the properties and reactivity of elements, designing new compounds, and predicting the outcome of chemical reactions.
- Physics: Explaining the structure of atoms, predicting the behavior of electrons, and developing new materials with desired electrical and thermal properties.
- Biology: Identifying the elements essential for life, understanding the role of metals in enzymes, and developing new drugs.
- Materials science: Designing new materials with specific properties, such as strength, corrosion resistance, and electrical conductivity.
- Education: Teaching students about the fundamental principles of chemistry and the properties of different elements.
Historical Significance
The development of the periodic table is a fascinating story of scientific discovery and intellectual triumph. The concept of organizing elements based on their properties was first proposed by several scientists in the early 19th century, but it was Dmitri Mendeleev who published the first widely recognized periodic table in 1869.
Mendeleev's table was revolutionary because it allowed scientists to predict the existence and properties of undiscovered elements. He left gaps in his table for these elements, and his predictions were later confirmed when new elements were discovered, such as gallium, scandium, and germanium.
The periodic table has undergone several revisions since Mendeleev's time to accommodate new discoveries and refine our understanding of the elements. Today, it remains an essential tool for scientists around the world.
: A Gateway to Scientific Discovery
The periodic table is a testament to the power of human curiosity and the beauty of scientific discovery. It's a gateway to understanding the structure and properties of matter, and it continues to inspire scientists and students alike to explore the mysteries of the universe.
Whether you're a seasoned scientist or just beginning your journey into the world of chemistry, this comprehensive guide to the periodic table will provide you with the knowledge and insights you need to unlock its secrets and unravel the wonders of the natural world.
4.1 out of 5
| Language | : | English |
| File size | : | 3515 KB |
| Print length | : | 66 pages |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Ashley Mcleo
Ashley Mcleo Arvind Narayanan
Arvind Narayanan Christopher D Winnan
Christopher D Winnan B Smith
B Smith B A Creatives
B A Creatives Baba Ifa Karade
Baba Ifa Karade Benjamin Kipkorir
Benjamin Kipkorir David Mitchell
David Mitchell Becky Cummings
Becky Cummings Gary Letcher
Gary Letcher V Totta
V Totta Jill Roman Lord
Jill Roman Lord Richard R Brettell
Richard R Brettell Beata Lubas
Beata Lubas Avery Carl
Avery Carl Geoffrey Saign
Geoffrey Saign Occupytheweb
Occupytheweb David H Levy
David H Levy Kris Harzinski
Kris Harzinski Patricia Bell Scott
Patricia Bell Scott
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Italo CalvinoThe Essential Guide to Customs Culture: Unraveling the Intriguing Tapestry of...
Italo CalvinoThe Essential Guide to Customs Culture: Unraveling the Intriguing Tapestry of... Christian BarnesFollow ·18.3k
Christian BarnesFollow ·18.3k Allen GinsbergFollow ·19.2k
Allen GinsbergFollow ·19.2k Finn CoxFollow ·8.9k
Finn CoxFollow ·8.9k Zachary CoxFollow ·2.2k
Zachary CoxFollow ·2.2k George BellFollow ·10.3k
George BellFollow ·10.3k Vincent MitchellFollow ·5.8k
Vincent MitchellFollow ·5.8k Heath PowellFollow ·10.4k
Heath PowellFollow ·10.4k Adrien BlairFollow ·3.7k
Adrien BlairFollow ·3.7k

 Ian McEwan
Ian McEwanWhy Didn't Anyone Say Anything? Uncovering the Hidden...
By [Author's...
 William Wordsworth
William WordsworthArthurian Legendarians: Faithless One - Part One – A...
In the realm of legendary tales, the...

 Corey Hayes
Corey HayesSSAT ISEE Prep Test: Arithmetic Review Flash Cards Cram...
Are you preparing for the SSAT or ISEE exam?...

 Robert Louis Stevenson
Robert Louis StevensonUnveiling the Essential Guide to Compliance: BCBS 239...
In the ever-evolving...

 Javier Bell
Javier BellJust Peachy: A Tale of Sweetness and Sassiness
Immerse yourself in a...

 Brent Foster
Brent FosterStep-by-Step Instruction Manual to Building a Real Estate...
Are you eager to embark on the...
4.1 out of 5
| Language | : | English |
| File size | : | 3515 KB |
| Print length | : | 66 pages |